

A Genuine Vascularization Solution
Better Biology by vascularizing your human-relevant models with ready to
use, isolated human microvessels.
Our Angiomics® microvessels are cryopreserved fragments of intact human microvessels (haMVs) recapitulating native microvascular biology and boosting physiological relevance to any 3D model. Use haMVs in existing, validated protocols or develop your own to integrate native microvasculatures into your cell and tissue models thereby enhancing discovery and redictability. Qualified for consistent performance, our haMVs combine microvascular cell complexity in a single reagent ready for immediate use. Realize better results by modeling
better biology with haMV vascularization.
Angiomics®
Human Microvessels
Different from just endothelial cells, Angiomics® haMVs are fragments of intact microvessels that are frozen in aliquots after a certified harvesting process and shipped in frozen cryovials. Our proprietary harvest process keeps intact the structure and cell composition of the native microvessel, including those cells residing in the perivascular niche.
When added to a 3D tissue or organoid model, such as the human vascularized MVP LIVER™ tissue model, the Angiomics® haMVs contribute to the tissue-specific environment, adding relevant and important complexity for better biological outcomes. Advance your research with a native microcirculation with the haMV-based Vascularized In Vitro Perfusion Model (VIPM) microphysiological system. Backed by decades of peer-reviewed research and comprehensive support, leverage Angiomics® haMVs to model angiogenesis, model vascularized tumor microenvironments, vascularize organoids/microtissues, and model perfused microphysiological systems

The presence of the Angiomics microvessels enabled more predictive treatment outcomes for in vitro cancer models.
Recapitulate Native Angiogenesis and Vascularization

Marker | % of Cells in Microvessel |
|---|---|
F4/80 | 23.60% |
CD2 | 0.80% |
Sca-1 | 11.40% |
Gr-1 | 5.10% |
CD146 | 28.20% |
CD34 | 26.70% |
Tie2 | 21.00% |
In a single haMV isolate, pieces of intact microvessels provide all vascular cells necessary to recapitulate native vascularization.
.png)
Tissue Vascularization
Tumor
Microenvironment
Vascularized
Organoids
Microphysiological Systems
Human Microvessels.
Angiomics hamV are intact microvessels that are frozen in aliquots after a certified harvesting process and shipped in frozen cryovials. The Angiomics hamV can be integrated into a tissue model with a variety of cells, 3D tissues and organoid assays to promote parenchyma performance resulting in better biological outcomes.
Backed by more than 50 peer-reviewed publications since 1996, the Angiomics hamV has been successfully researched and applied in angiogenesis dynamics, angiogenesis and tissue biomechanics, microvascular biology in health and disease, tissue vascularization and graft/implant vascularization.

50-200µ in Length
10-50µ in Diameter

Vascularize with Confidence
Protocols for using haMVs are provided with each vial and scientific expertise is available to support your vascularization outcomes.
Model Diseases.
Combine with the BioAssemblyBot® and BioApps® Platforms to automate your vascularized tissue modeling and assaying in order to save time, reduce costs, and streamline your research.

Ready for immediate use
Our haMVs are delivered frozen in standard vials containing different numbers of microvessels tailored for different applications. Thaw and use the microvessels however you would use cells to vascularize your models.
Our unique approach to vascularizing tissue:
Recapitulate angiogenesis within a wide variety of 3D culture environments.

Integration
Microvessel vials are thawed and pipetted or bioprinted into 3D cell culture.
Neovessels sprout from parents and retain lumens.
Incubation
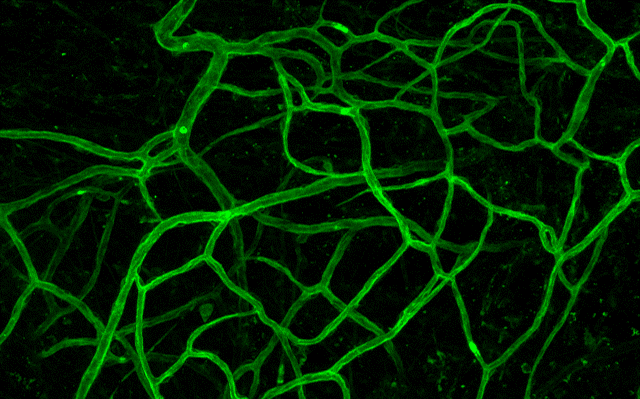
Inosculation
Growing vessels inosculate, establishing a perfusable microvasculature.

Angiomics Key Features & Specifications:
Native Human Microvessels
Parenchyma/Stromal Cell
Microvessel Cooperativity
Full Complement of
Microvascular Cells
Recapitulates In Vivo Vascularization
Phenotypically Plastic & Dynamic with the Tissue Environment
30+ Years
of Tissue Vascularization
Expertise and Support
Preparation & Packaging:
Vials are frozen at the time of isolation and stored in liquid nitrogen
Growth Potential:
Each microvessel lot is qualified for positive neovessel growth activity
Microvessel Source:
Microvessels are isolated from subcutaneous adipose.
Microvessels are isolated from donated human tissue for use in research by informed consent or legal authorization. Age and sex of donor are provided with each lot.
Available Sizes:
20K, 50K, or 100K microvessels/vial
Angiomics Human Adipose Microvessels by Advanced Solutions are for RESEARCH USE ONLY and not for use in humans under any circumstances. Advanced Solutions Life Sciences, LLC. and Advanced Solutions, Inc. are not responsible or liable for how this product is used. The RESEARCH USE ONLY limitation supersedes any written, oral, or implied understanding between the parties.

Ready to get started?
Book a demo, get a quote, or connect with a vascularization expert:
Angiomics Human Adipose Microvessels by Advanced Solutions are for RESEARCH USE ONLY and not for use in humans under any circumstances. Advanced Solutions Life Sciences, LLC. and Advanced Solutions, Inc. are not responsible or liable for how this product is used. The RESEARCH USE ONLY limitation supersedes any written, oral, or implied understanding between the parties.

The Advanced Solutions Louisville, KY facility has been audited by UL Registrar LLC and meets GMP requirements listed in RCP, NBCP, or Pharmaceutical Certification Schemes which is uniquely accredited by the American National Standard Institute (ANSI) developed in accordance with applicable sections of the FDA’s code of federal regulations.
